U.S. and Canadian mammogram industry spends $3 billion annually on breast cancer screening. Worldwide = $75.1 billion USD by 2025 -marketsandmarkets.com
Breast cancer claims the lives of almost 50,000 women in the US each year.
Industry Demand: An estimated 100 million people in North America are in the primary target group for breast cancer screening. 50,000 women each day are added to that group. For more than 20 years, mammography x-ray screening became the standard breast cancer detection method in USA and Canada.
Mammofield® experimental bioelectric field-based technology offers the possibility to be a reliable harbinger of cell-tumor screening. Detection system is non-contact, non-radiative, and non-invasive, being in a comfort zone for all patients. The potential mammary map produced by Mammofield® could possibly be both accurate and to cell level, allowing pinpoint accuracy, thus not allowing subjective conclusions to be drawn from observed data, a deficiency in x-ray mammography. Early cell tumor development detection
is the asset of Mammofield®, compared with x-ray screening’s sometimes tumor-decade-life before-detection period. No one else has this proprietary technology of non-contact detection and identification of cancer cell bioelectric fields.
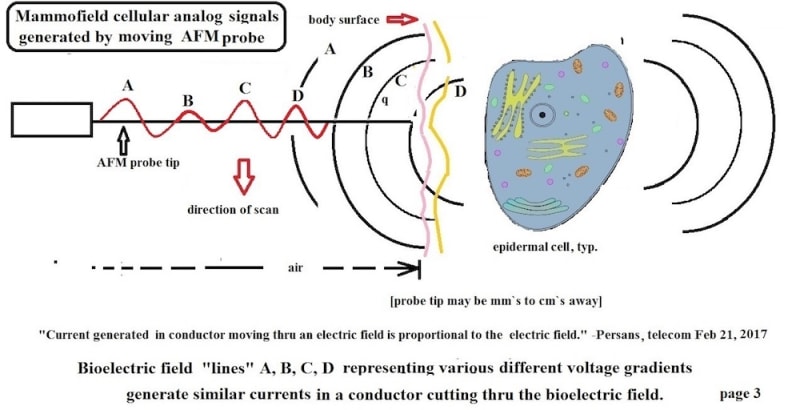
The present product, “Mammofield” scans a person`s an area to be studied. Preliminary results using non-contact passive AFM probes with tip radii of 1-3 microns show detailed data stream which can be searched for cell field potentials and currents. Earlier research with 0.001 inch probes also show some data stream for analyses.
Early and present experimental prototypes utilize motor - driven scanning. Future prototypes could utilize very fast rail or solid-state array scanning. This will result in a more accurate instrument. Larger experimental prototypes can be built to enable full-body scans in clinics, hospitals and research facilities. The probe width determines the cell size scan; the length determines the detection depth.
These larger Mammofield units would also be used to gain insight via research into internal organ cell fields. It would be produced by a medical device manufacturer under ISO FDA approved methods and facilities. Every part is off-the-shelf, thus enabling low cost of manufacture at a 2000-to-one price /cost ratio.
It was recommended for breast, skin and tissue cancer research by 2 University of California cancer researchers after 3 years` experiments [one oncologist, one eye cancer specialist]; have one FDA regulatory expert, one M.S.E.E. Have large pension fund on board with matching co-investment.
Mammofield was recommended for melanoma clinical trials and also for early warning transplant organ failure by an Albany Medical College research oncologist.
Competition: TransScan has a non-invasive, non-radiation T-Scan product utilizing a multi-electrode sensor array pressed against the breast for detection of breast cancer based on TransSpectral Impedance Scanning. This is uncomfortable but has resulted in increased detection rates. There are currently four digital mammography manufacturers: General Electric, Fisher, LoRad, and Fuji. Standard x--ray mfg’s are: Advanced Instrument Development, Faxitron X-Ray Corporation, Fischer Imaging Corporation, GE Medical Systems, Instrumentarium Imaging, Inc., Philips Medical Systems and Toshiba America Medical Systems. Magnetic resonance imaging (MRI), ultrasound and thermal imaging are also used.
Video
Like this entry?
-
About the Entrant
- Name:William Olivadoti
- Type of entry:individual
- Software used for this entry:off-the-shelf commercial sw used
- Patent status:pending