Cor-IS is a portable, non-invasive device for the diagnosis of Coronary Artery Disease (CAD) through brachial artery endothelium functionality evaluation. Until now, ultrasound imaging of the brachial artery is applied in clinical practice, but this has major disadvantages like poor repeatability, extensive fluctuation in the measured values, low imaging resolution and being dependent on ultrasound operator. These drawbacks are not present when employing Cor-IS. Cor-IS applies space technology developed for sensing bubbles presence in the human body of astronauts during Decompression Sickness. The device is based on an EU patented electrical impedance spectroscopy technique. Recently, an application has been submitted for Cor-IS intellectual properties protection in Europe as regards impedance measurements correlation with CAD diagnosis. Thus, Cor-IS has reached TRL 4 and targets to TRL 7 or 8 in 2018. Cor-IS evaluates the functionality of the brachial artery endothelium and, consequently, of the coronary circulation system.
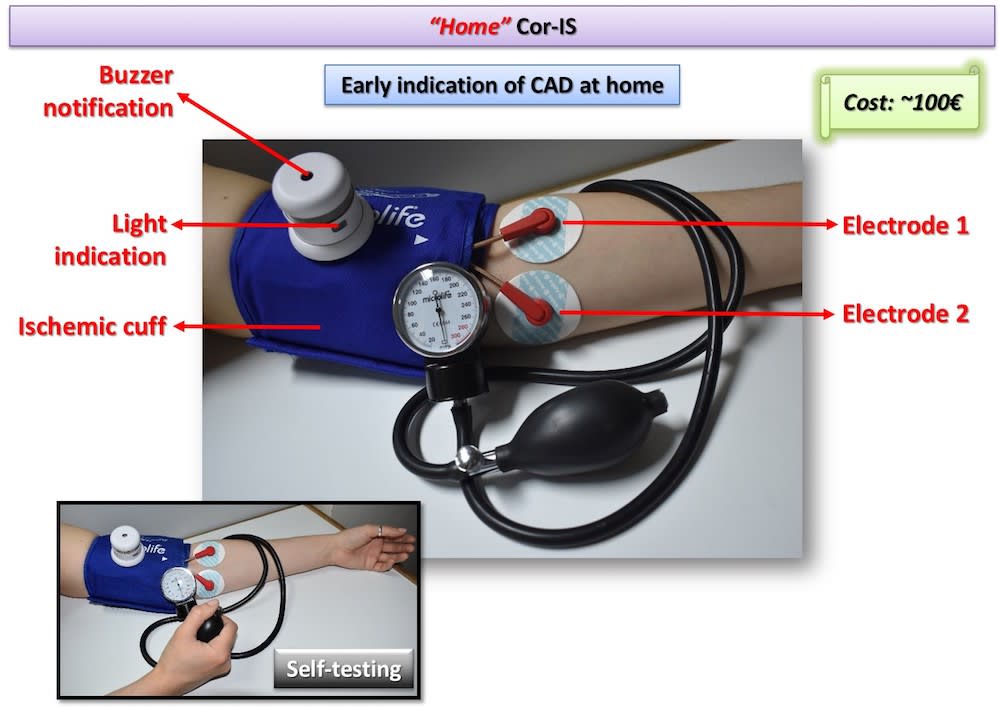
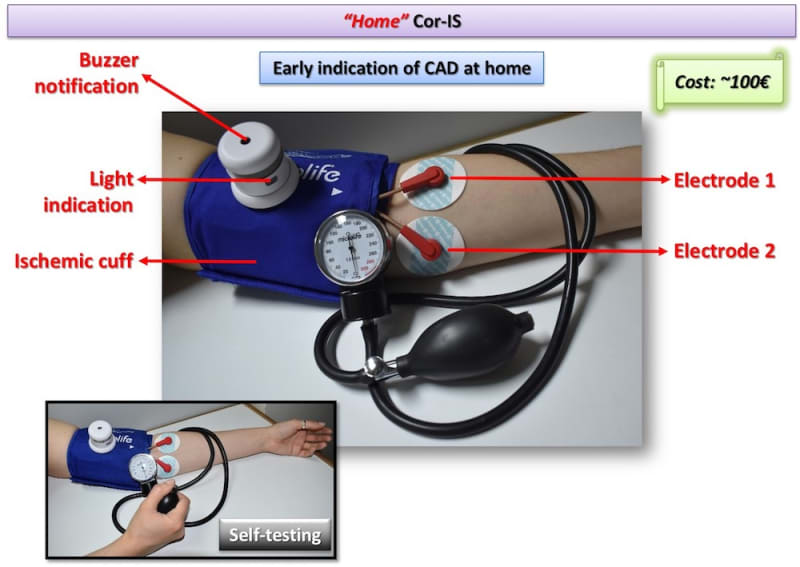
The device consists of the data acquisition/processing unit which is connected with a cuff and a pair of electrodes which are placed to pre-selected positions of the left hand. Cor-IS measures for varying excitation frequency values the electrical impedance response of brachial artery. The measurement lasts 15 minutes and is divided in three periods: 1st (5 min): Baseline impedance measurement, 2nd (5 min): Application of ischemic cuff that increases measured impedance, 3rd (5 min): Subsequent vasodilation and hyperemia through the Nitric Oxide release from the endothelium that decreases measured impedance. Cor-IS employs innovative methods for a) identification of proper excitation signal parameters, b) signal acquisition and c) digital signal processing that improve measurement sensitivity two orders of magnitude compared to conventional electrical techniques. Moreover, acquired signals are analyzed by means of custom-made, advanced algorithms in both time and frequency domain. Signal analysis focus mostly on the temporal variation of measured impedance, the transition phase from ischemic cuff to hyperemia and specific frequency components.
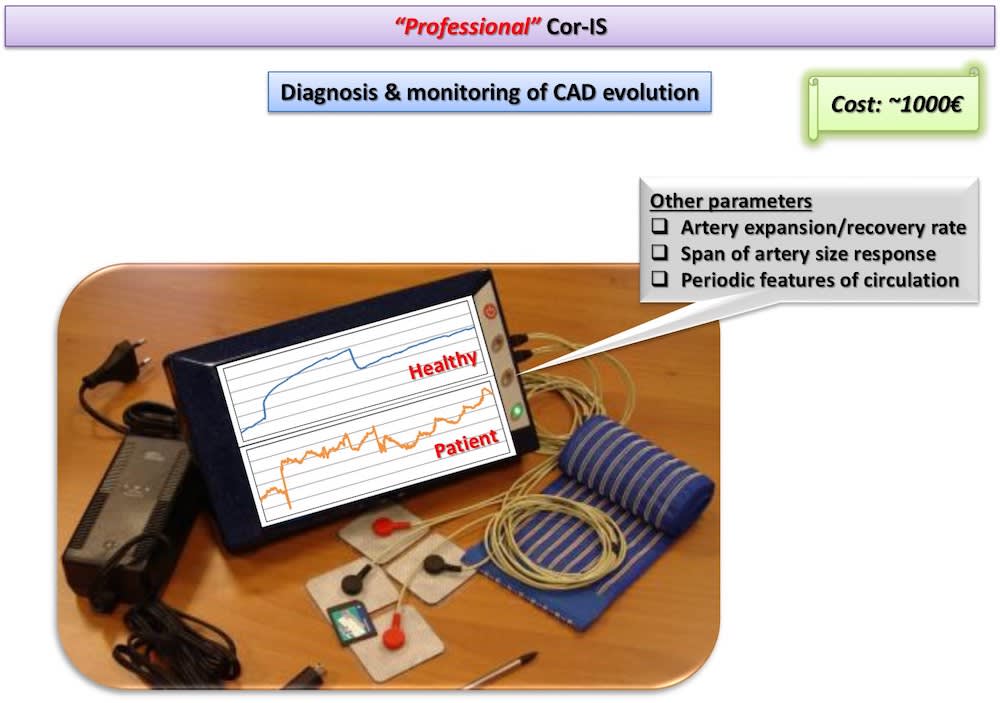
Results concern, primarily, the early diagnosis of an emerging CAD in patients suspected to develop the disease and, secondly, the determination of parameters such as brachial artery expansion and recovery rate, span of artery size response, periodic and non-periodic features of coronary circulation, etc. Such information will allow medical doctors to monitor the CAD evolution along patient’s lifetime.
Two Cor-IS versions are currently under development: a) The “home” version for the early indication of an emerging CAD at home and b) The “professional” version for both diagnosis and monitoring of CAD evolution. It is worth-noticed that telemedicine applications can be integrated in the device for use in remote areas. Cardiovascular disease diagnostics market will reach 7 billion $ by 2018. Cor-IS device is very promising and its introduction to the market is considered feasible since ultrasound devices cost more that 30k€ while “home” Cor-IS will cost ~100€ and “professional” version ~1000€.
-
Awards
-
 2017 Top 100 Entries
2017 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Sotiris Evgenidis
- Type of entry:teamTeam members:Prof. Thodoris Karapantsios (Chemical Engineer) Dr. Sotiris Evgenidis (Chemist) Dr. Konstantinos Zacharias (Electrical Engineer) Dr. Giorgos Karagiannis (Cardiologist)
- Patent status:pending