There are over 1 million hernia repairs annually in the United States, the majority of which are inguinal hernias (approximately 800,000). Our understanding of hernia pathophysiology has improved so we currently have moved away from the traditional tissue-based repairs towards open/laparoscopic/robotic tension free mesh-based repairs. Many prospective randomized trials have favored outcome of mesh-repair of abdominal wall and inguinal hernias compared with primary repair confirming lower recurrence rates, decreased post-operative pain, and shorter hospital stay.
Different mesh shapes and material which are all solid and different surgical techniques are currently available and are used for hernia repairs but none are ideal. We are still far away from having an ideal repair technique and hernia repair concept. Current commercial meshes are either permanent or made of bio-absorbable plastics. Deficiencies of current commercially available meshes include: 1) requiring high mesh handling time, 2) increased operation time (to measure and choose the appropriate mesh and place/fix the mesh), 3) high infection rates due to mesh exposure to skin and outside environment, 4) high amount of wasted mesh as excessive mesh is discarded, 5) the need to stock different sizes and shapes of various meshes, 6) difficulty with placement of mesh in open/laparoscopic /robotic techniques, 7) difficulty with measuring required mesh size in the closed body cavities, 8) high rate of mesh clips/sutures / tuckers/glue misplacement cause recurrence and long term pain (nerve impingement), 9) occasional inflammatory reaction to the mesh, and 10) suture failure which could have multiple consequences including mesh delayed move and malposition causing recurrence and chronic pain.
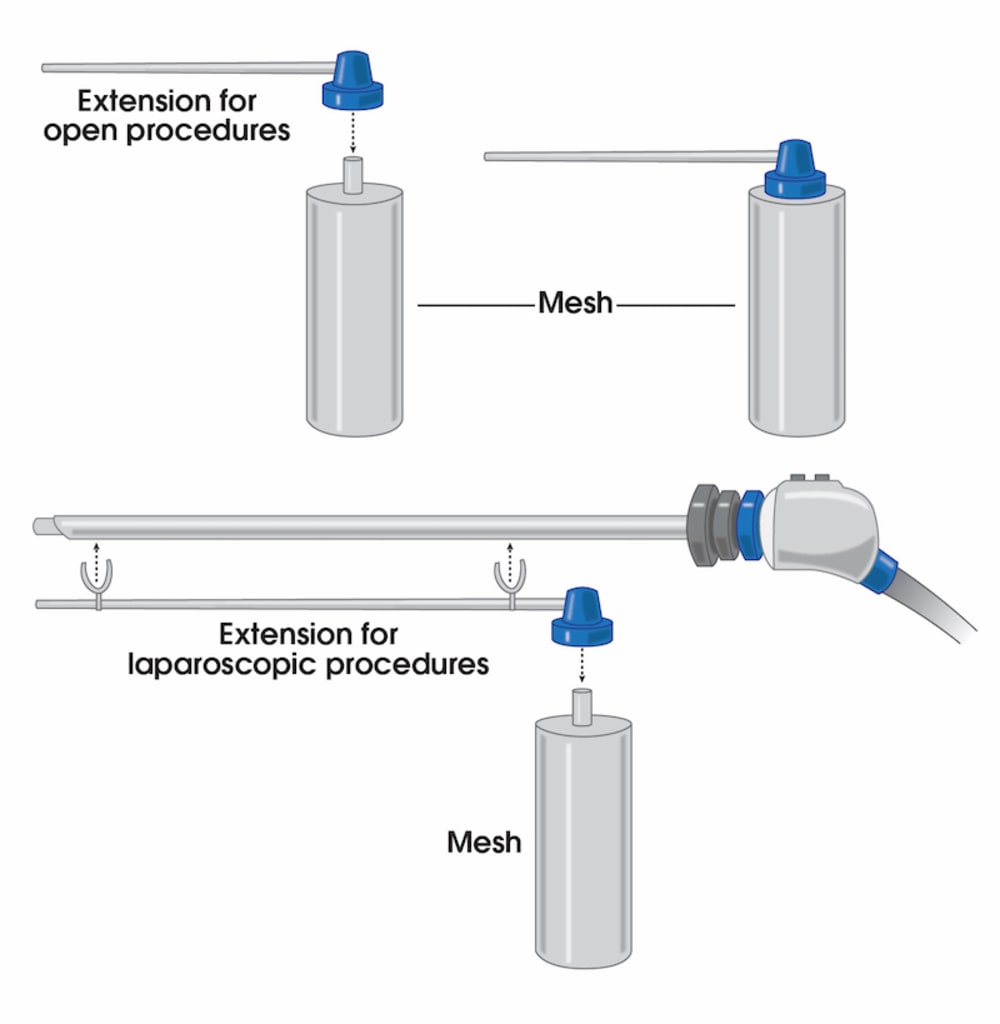
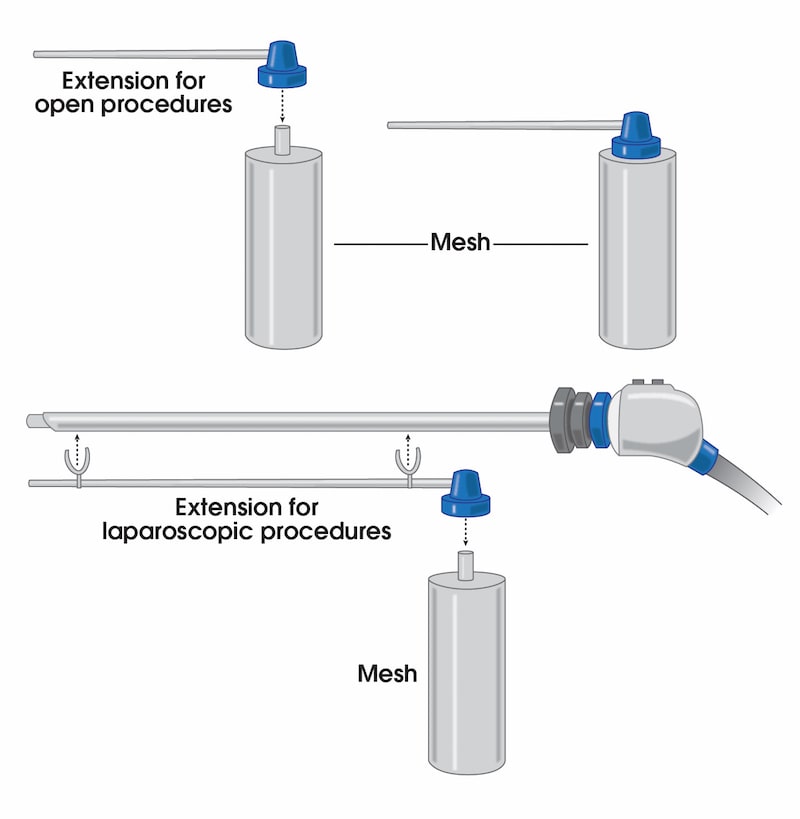
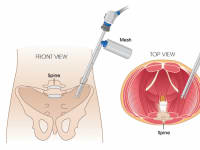
To address these issues, we have introduced a novel liquefied mesh delivery system. The Mesh Sprayer Device (MSD) affixes liquefied sterile mesh into the abdominal wall and thoracic defects. MSD delivers a layered homogenized liquid which will change into a solid/semi-solid form following delivery and creates a medium for reinforcement of the abdominal wall and viscera at the site of entry and functions as an alternative to currently available surgical meshes. The spray canister can be fitted over a laparoscopic/robotic camera or other instruments. MSD can be used for placement of mesh in the open repairs too which includes spraying a layer of mesh over the required surface. MSD is usable in every case of abdominal surgery and by laying an extra layer of support will simply prevent the risk of future hernia formation at the site of facial closure. MSD reduces the mesh handling time, operation time, mesh contamination, wasted material, wounds infection rates, and the costs to a minimum and will ultimately improve the patient satisfaction. The placement of this mesh for open, laparoscopic, and robotic techniques are very easy and includes only one step of spraying the surface. MSD does not need precise size measurements and it eliminates the need for clips/sutures/tuckers/glue placement and potential misplacement. MSD would potentially reduce the risk of early or late hernia recurrence and or associated long-term pain associated with mesh or clips/sutures/tuckers/glue misplacement.
Currently provisionally patented through LSUHSC-S-2017-003 with reference 62/476, 926.
-
Awards
-
 2017 Top 100 Entries
2017 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Alireza Hamidian Jahromi
- Type of entry:teamTeam members:Alireza Hamidian Jahromi, MD, General Surgery Resident, LSU Health Shreveport, LA; David Ballard, MD, Radiology Resident, Washington University, MO.
- Software used for this entry:no
- Patent status:patented