Procyrion, Inc. of Houston, TX is developing the first catheter-deployed heart pump intended for long-term treatment of chronic heart failure. Thinner than a #2 pencil and only 6cm long, AortixTM has the potential to become a low-risk circulatory assist device for a broad range of patients.
Heart failure accounts for 3.6 million hospitalizations, 300,000 deaths, and $35 billion in annual healthcare costs in the US alone. More than 3 million heart failure patients currently suffer from chronic fatigue and painful swelling while performing normal activities of daily living but cannot be managed by medication alone and have no safe and effective therapeutic option.
The only treatments available to these patients are heart transplants, which are limited by scarcity, and surgically implanted pumps called ventricular assist devices (VADs), which are so risky, invasive, and expensive they are only used as a last resort. This leaves more than 2 million people in the US with no choice but to suffer a slow decline in quality of life.
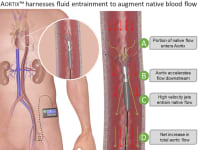
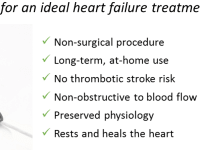
Procyrion aims to give heart failure patients a solution with Aortix, a powerful micro-pump designed to rest and heal the heart without surgery and with minimal risk. Aortix is percutaneously deployed in a simple 10 minute catheter lab procedure. First, a cardiologist guides the pump through a deployment sheath in the femoral artery to the descending thoracic aorta. Once in place, the sheath is retracted, allowing self-expanding nickel-titanium struts to deploy and anchor the pump to the aortic wall.
Aortix augments heart function by accelerating a portion of native blood flow within the pump and pushing it downstream to entrain aortic flow. Strategic placement downstream of the heart allows for combined benefit to the heart, kidneys, and other vital organs while eliminating the common VAD risks of damage to the heart and thrombotic stroke. Furthermore, Aortix is the first catheter-based pump suitable for long-term ambulatory use and is non-obstructive to native blood flow, unlike current VADs in which device failure is often fatal. The end result is reduced afterload, the heart working at a sustainable level, and healthy blood flow and pressure throughout the body.
In its current configuration, Aortix is powered through a flexible, transdermal lead that attaches to a pocket-sized micro-controller. The system can operate for more than 8 hours on a single charge, but external battery packs are “hot swappable”, meaning patients could charge or replace batteries without the risk of pump failure.
Aortix is a first-in-class device that holds the promise of extending the lives and dramatically improving the lifestyles of millions of heart failure patients around the world. It represents a win for multiple stakeholders: the cardiologist gains a safe and effective treatment option where none currently exist; the hospital converts lengthy, money-losing intensive care visits to profitable outpatient procedures; the insurance company sees a reduction in payments due to improved patient health; and, most importantly, the patient receives low risk, minimally invasive treatment early enough in the progression of heart failure to truly alter its course.
Video
-
Awards
-
 2015 Grand Prize Winner
2015 Grand Prize Winner -
 2015 Top 10 Most Popular
2015 Top 10 Most Popular -
 2015 Top 100 Entries
2015 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Omar Benavides
- Type of entry:teamTeam members:Procyrion, Inc. Team Members:
Benjamin Hertzog, PhD - President & CEO
Jace Heuring, PhD - COO
Reynolds Delgado, MD - CMO
Will Clifton, MD - Director of R&D
Omar Benavides, PhD - Sr. Product Dev. Engineer - Software used for this entry:SolidWorks; LabView
- Patent status:patented