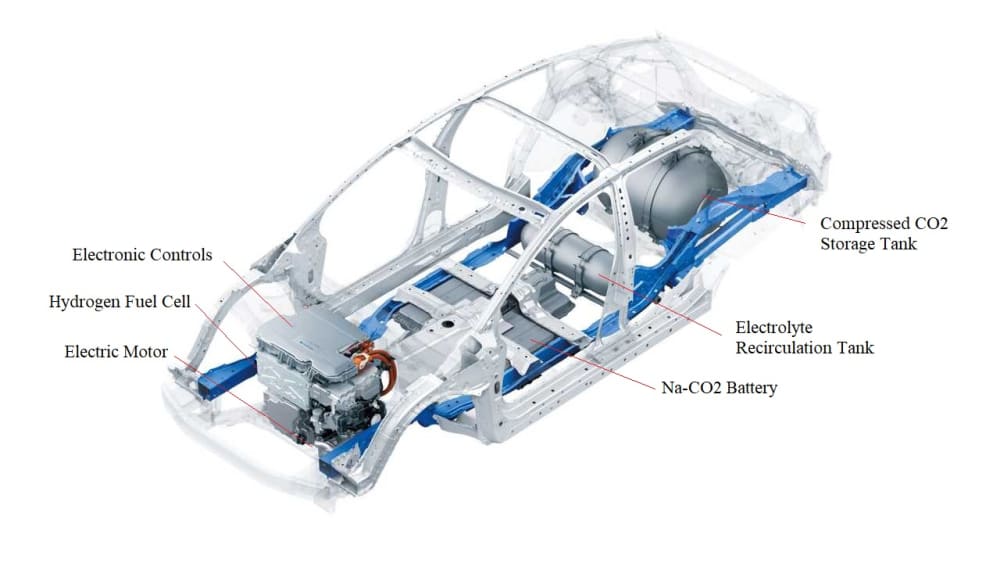
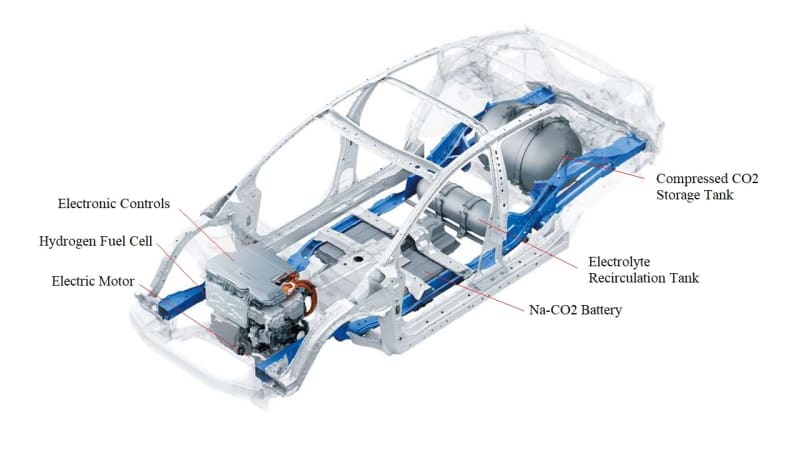
The International Panel on Climate Change (IPCC) estimates the need at approximately 10 gigatonnes of net CO2 removal per year by the year 2050 in order to keep global temperature rise under 1.5 or 2C. There is a tremendous need to have perennial and continuous access to cost-effective electricity generated from the intermittent energy sources (wind, solar, geothermal, hydropower, wave, etc.). This will require development of inexpensive and efficient electrical energy storage (EES) devices such as stationary batteries for uninterrupted electricity (power storage back up) and load leveling and electric vehicles (EVs). There is also a tremendous need for systems capable of carbon capture and sequestration. This solution will demonstrate net-negative and durable CO2 sequestration using a Mg-CO2 battery EES System with hydrogen production and utilization. The CO2 is captured by the Mg-CO2 battery and permanently sequestered by chemical transformation.
Imagine a battery that stores energy and consumes Carbon Dioxde (CO2) as the fuel to produce hydrogen gas. This is the idea behind our innovation - a solution to global problems such as EES and carbon sequestration. We start with a Mg/CO2 battery, like all batteries, it stores electricity, however, it also produces hydrogen gas as a by-product of operation. We propose to capture the hydrogn gas from the batteries and feed the gas into a Fuel Cell to produce additonal electricity. The combined energy out-put can be used in a wide variety of applications including EVs. Welcome to the Carbon Dioxide to Hydrogen economy.
Mg-CO2 batteries can theoretically store 10 times as much energy by weight as commercial lithium–ion batteries. With an energy density of about 1000Wh/kg, Mg-CO2 batteries have the potential to surpasses current commercial counterparts. Lithium-ion batteries that are used in Tesla electric cars have only about 180Wh/kg of energy. The energy storage capability of Mg-CO2 batteries is very important as they potentially have high energy storage capability, but there is another overlooked advantage - hydrogen production. The production of hydrogen in metal ion batteries using water based ectrolyte is a natural process. In the past battery designers worked hard to surpress hydrogen generation using a variety of chemicals. Our innovation is to take advantage of the batteries natural hydrogen production to feed a fuel cell, thereby increasing over all energy storage and production of new energy.
Metal Ion batteries can produce a large quantities of hydrogen gas during operation. This hydrogen gas can be captured and utilized in a fuel cell to not only store energy but produce additional energy. Using a Mg-CO2 battery with water based electrolyte we are able to create a CO2 to hydrogen economy. CO2 is injected into the water in the battery which causes the water to become acidic, thereby, allowing ions to pass through the water to the cathode. The electric potential will generate hydrogen and oxygen by electrochemical decomposition of H2O. The oxygen is consumed in the formation of Magnesium Carbonate (MgCO3) leaving the Hydrogen free for extraction and use.
-
Awards
-
 2022 Top 100 Entries
2022 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Danny Jones
- Type of entry:individual
- Patent status:pending