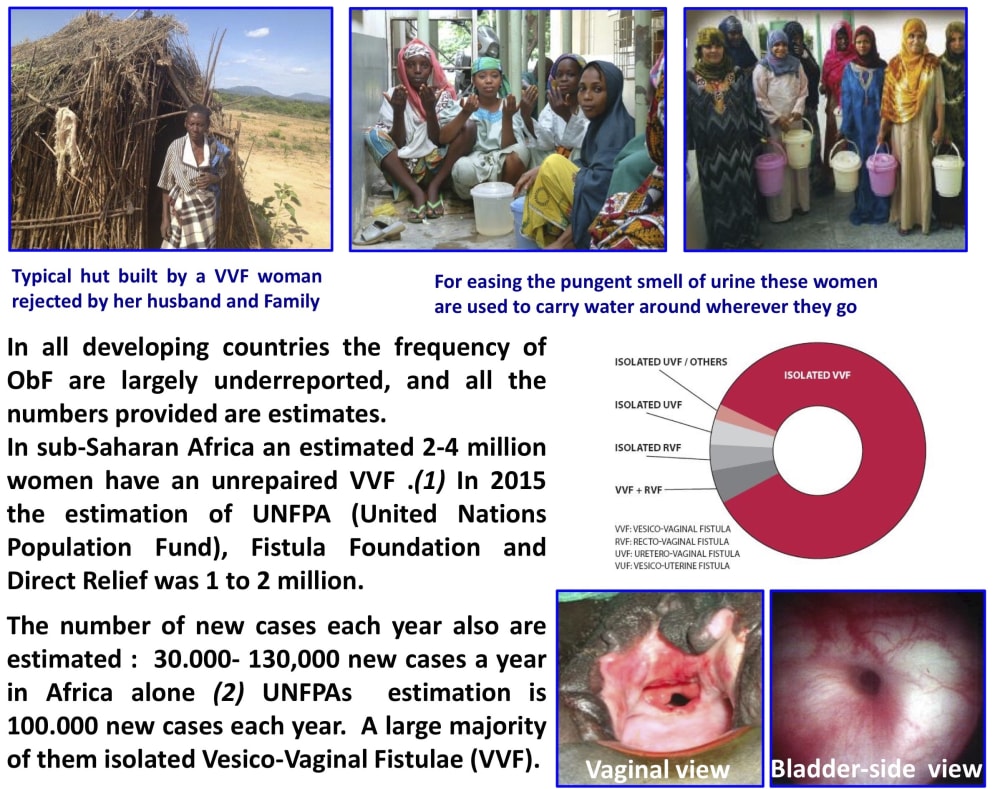
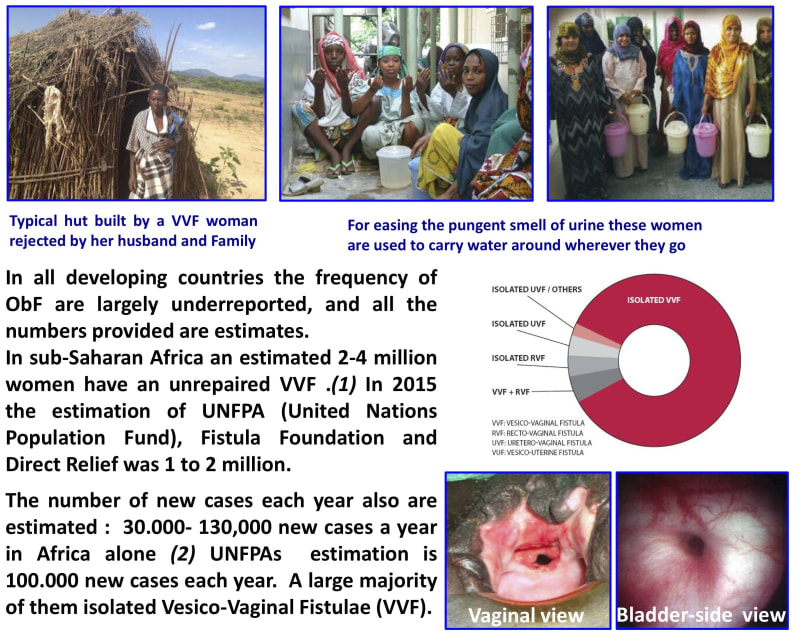
One woman with Obstetric Fistula (ObF) said ‘Cure my leaking fistula first. If I am blind, people will sit with me and talk to me, but no-one will come near me because I’m wet and I smell..’
One to four million marginalized young women worldwide are affected by ObF. Fistulas develop during obstructed labor (this is a medical term for describing passage of the fetus through the birth canal), often a result of childbirth before a woman’s body has fully developed, during her early teens. The baby cannot pass and dies during a birthing process that can take days. As the mother’s uro-genital tissues are compressed pressure necrosis develops, creating an opening between the bladder and the vagina and/or rectum. After miscarrying, women with ObF continuously leak urine and have a persistent smell. They are ostracized by their friends and family, their chances of working or being part of their community are almost non-existent. Left untreated it leads to chronic incontinence, and can cause kidney infection and painful sores.
Worldwide there is a limited surgical capacity to treat ~18,000 fistulas at a rough cost of $400 per procedure. Every year there are 60-130,000 new cases. Instances of obstetric fistulas are infrequent in the developed world, with child-marriage a rare taboo. It is sadly common in areas already stricken by poverty and underserved by the few surgeons skilled enough to successfully operate. These marginalized and hidden women cannot all be treated, but they can be helped.
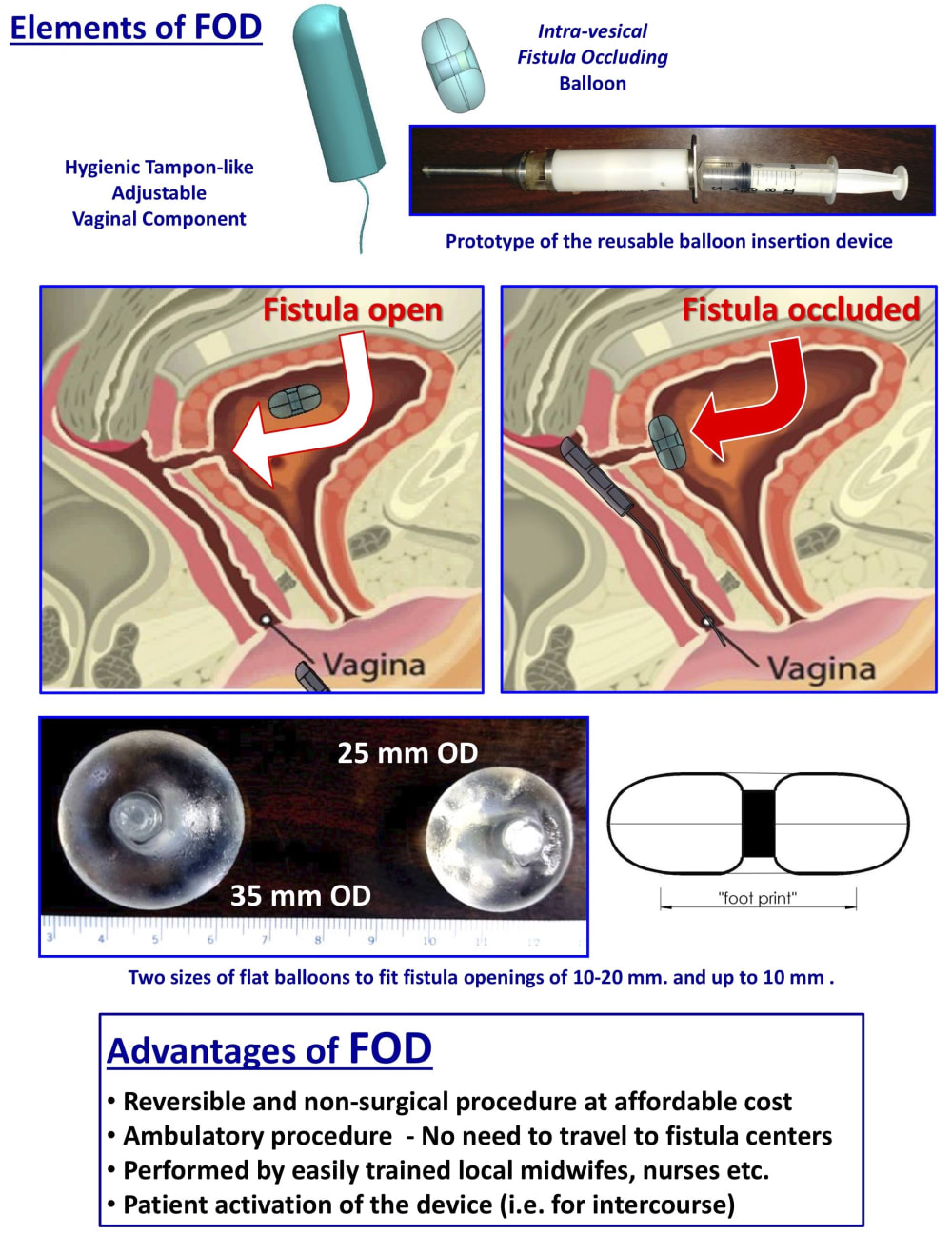
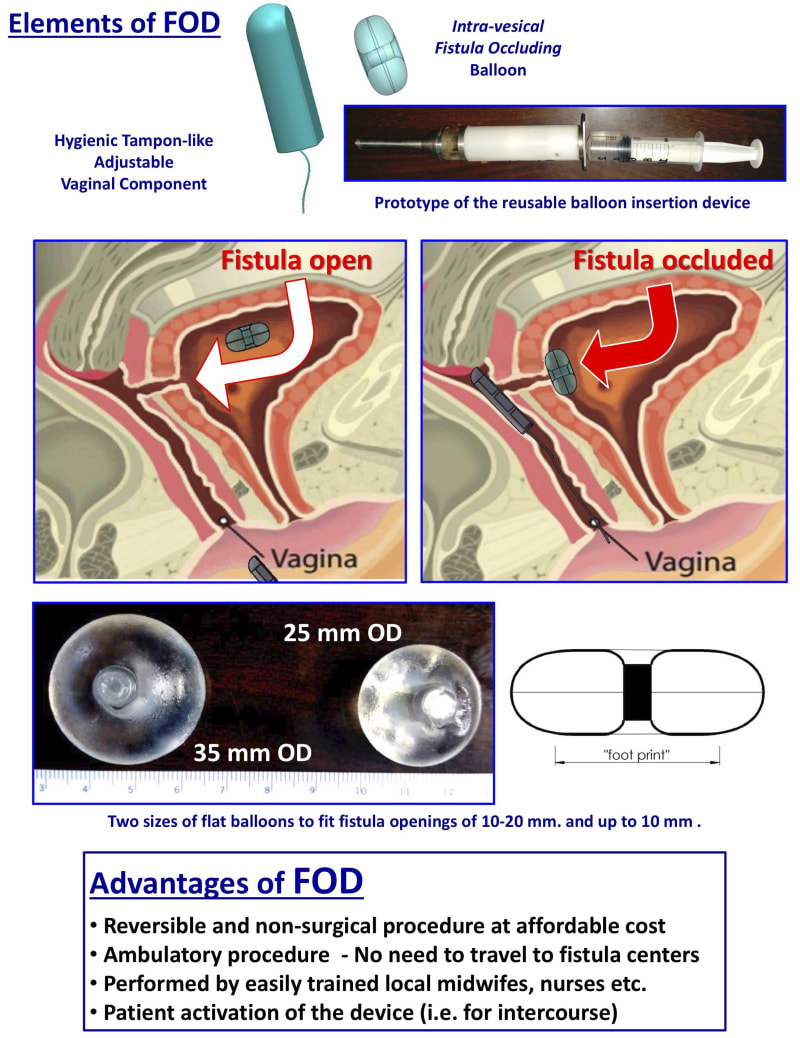
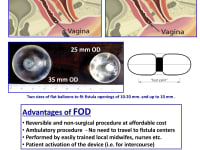
The Fistula Occluding Device (FOD) developed by Innoventions LTD. is a life-restoring palliative treatment that mechanically occludes the fistula. It is an affordable, reversible procedure that can be performed by local nurses and midwives with minimal training.
The FOD comprises a special intra-vesical occluding balloon containing a magnet and a removable silicone encased vaginal magnet, similar to a tampon. It is a simple treatment where the magnetic force of the vaginal insert attracts the balloon in the bladder to occlude the fistula, thereby creating instant dryness and allowing women to regain their lives.
The silicon balloon is inserted in a non-surgical procedure via the urethra with an Inserter. It remains within the bladder, for 3 - 6 months and is removed with a Retriever and then replaced. These devices will be produced en-masse either using plastic injection molds, or 3D print processing using medical grade plastic that can be autoclaved.
The Inserter and Retriever are completely reusable, only the balloon containing the capsule and the pusher are single use. The devices are simple-to-use and mistake-proof.
The incontinence, shame and isolation of Obstetric Fistulas in developing countries can be stopped with the FOD.
Like this entry?
-
About the Entrant
- Name:Benjamin Boyarsky
- Type of entry:teamTeam members:Dr Daniel Yachia - Chief Medical Officer
Ofir Yulish - Engineer - Patent status:none