Angina is a form of chest pain caused by reduced blood flow to the heart and is a symptom of coronary artery disease. It is often described as squeezing, heaviness, tightness, or chest pain. Refractory angina (RA) is a persistent condition of angina, which cannot be treated by current medical therapy, angioplasty/percutaneous interventions, or coronary bypass surgery. It causes persistent pain, psychological distress, and activity limitations, which can significantly impact a person’s quality of life. However, no effective treatment has been developed to address it. RA occurs in patients who are not good revascularization candidates as well as in a portion of patients following successful revascularization. Angina may persist following revascularization, with prevalence as high as 25% after 1 year and up to 45% after 3 years. Unlike stable angina, RA does not respond to medication and is debilitating. Physical activity of RA sufferers is severely limited, and they often suffer from long-term coronary artery disease and are at a greater risk for heart attack. This often leads to an increase in stress which can further exacerbate their condition.
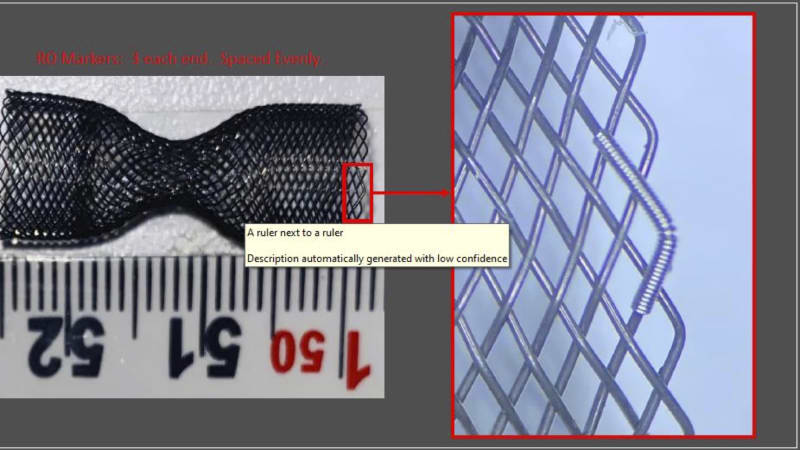
Recently, coronary sinus flow reduction has been introduced as a safe and effective treatment for RA. In various clinical studies in Europe, Israel and UK, our competitor, it has been shown that coronary sinus reduction alleviates symptoms and improves RA patients' quality of life. However, the sole coronary sinus reducer on the market has design drawbacks which limit the population of RA sufferers that respond to treatment as well as add risk to the clinical workflow of the procedure. VahatiCor is pursuing a fast follower strategy to address the RA market but with a next-generation device which overcomes the design limitations of the competitive technology.
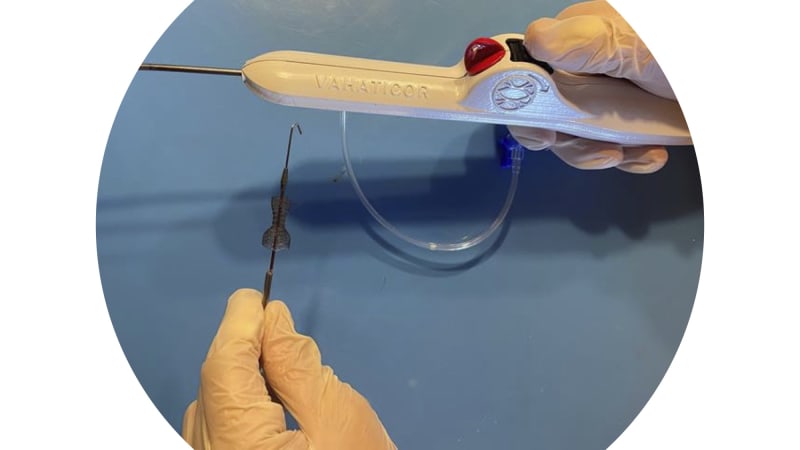
- Self-expanding reducer device and delivery system developed and extensively evaluated
- Regulatory pre-submission provided to the FDA
- Several strategic parties assessing VahatiCor’s technology
- Chronic and acute animal studies already initiated at the Cleveland Clinic
- Differentiated intellectual property for reducers and temporary occlusion devices in the coronary sinus
- Filed and FTO completed in patients who
Like this entry?
-
About the Entrant
- Name:Howard Edelman
- Type of entry:individual
- Software used for this entry:N/A
- Patent status:pending