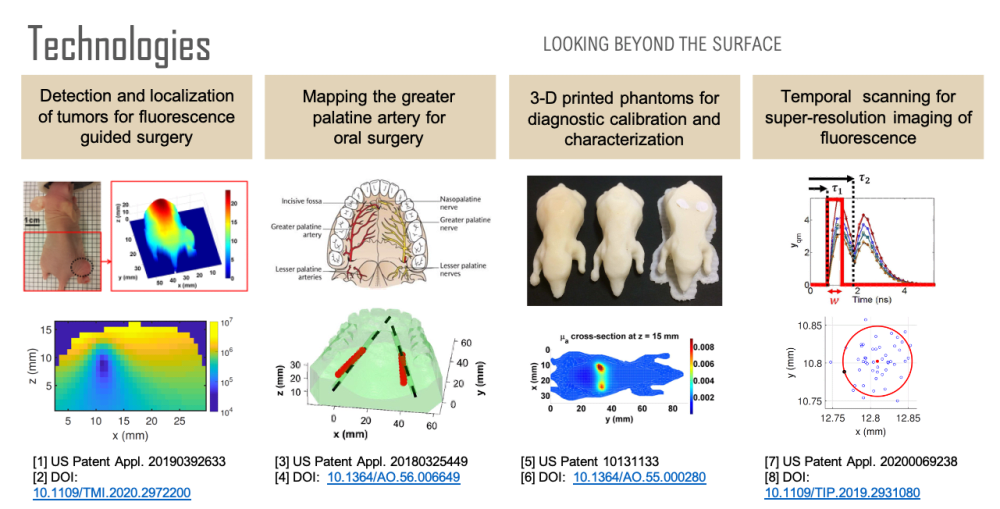
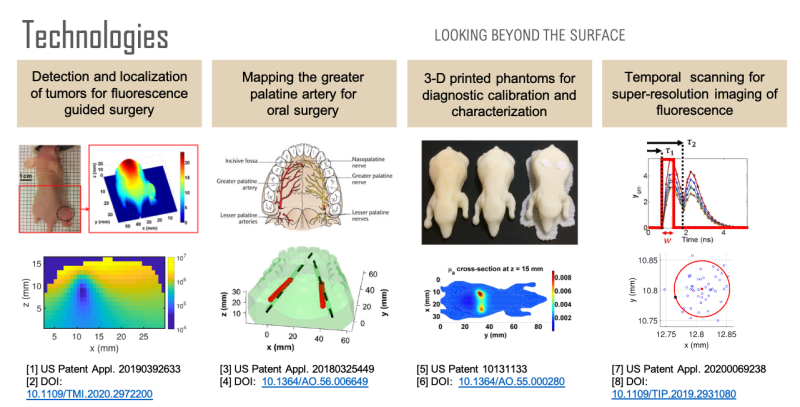
Non-invasive imaging at centimeter depths in tissue is possible with computational diffuse optical imaging (DOI). Camera measurements of scattered light at the tissue surface are interpreted with a light transport model to reconstruct optical parameters within the tissue, providing contrast for object detection, localization, and identification. We have developed a suite of technologies based on these concepts to assist in surgery with the goal of reducing complications and improving surgical design.
First, over forty percent of human cancer cells over-express folate receptors such that the cells can be identified with folate-targeted fluorophores, enabling fluorescence guided surgery. A previous study has shown that a surgeon can detect 5 times more malignant masses with the aid of fluorescence than with the naked eye. However, once a tumor has been identified, quantification of its size and location could be used to reduce margin and damage to surrounding healthy tissue during tumor removal. Using a mouse model, we have developed a technology for the localization and characterization of a tumor to assist in fluorescence guided surgery.
Second, oral surgeons use the greater palatine artery (GPA) in the roof of the mouth as a landmark and need to know where it is to avoid damaging it or the surrounding nerves, for example, when harvesting tissue. However, studies have shown that there can be a discrepancy of 4 millimeters between where surgeons believe the artery is and where it actually is. Significant bleeding can occur if the GPA is severed, especially if the artery retracts into the bony canal making it difficult to access. Using a tissue phantom model, we have developed a technology for determining the location of the GPA to assist in oral surgery.
Third, none of the available in vivo imaging methods provide direct access to neurons. For example, fMRI measures the blood-oxygen-level-dependent (BOLD) contrast signal, a secondary indicator of neuron activation. Direct access to neurons in vivo could be considered a holy grail in brain science. We have developed a technology that offers a possible path forward. Neuron activity involves Ca2+ transport through channels in the cell body. Monitoring Ca2+ signaling with Ca2+ fluorescent reporters thus presents a direct picture of neuron activity. We have developed a super-resolution localization imaging concept based on spatiotemporal modulated data for finding individual neurons throughout the brain. Applying the method in a mouse model is ongoing work.
Each of these sensing capabilities is developed and calibrated using 3D printed tissue-simulating phantoms, a technology we pioneered. 3D printing is ideal for fabricating phantoms because objects with virtually any desired shape can be built. By printing with multiple materials, it is also possible to place complex inhomogeneities at desired locations within the phantom. Such phantoms are useful for developing and calibrating measurement setups, characterizing spatial resolution and depth penetration, optimizing the signal to noise ratio, and comparing performance between systems. Furthermore, a phantom can simultaneously be used for calibration and as a surgical guide that occludes tissue regions that a surgeon should avoid.
Like this entry?
-
About the Entrant
- Name:Brian Bentz
- Type of entry:teamTeam members:Kevin J. Webb, Professor of Electrical and Computer Engineering, Purdue University
- Software used for this entry:MATLAB
- Patent status:patented