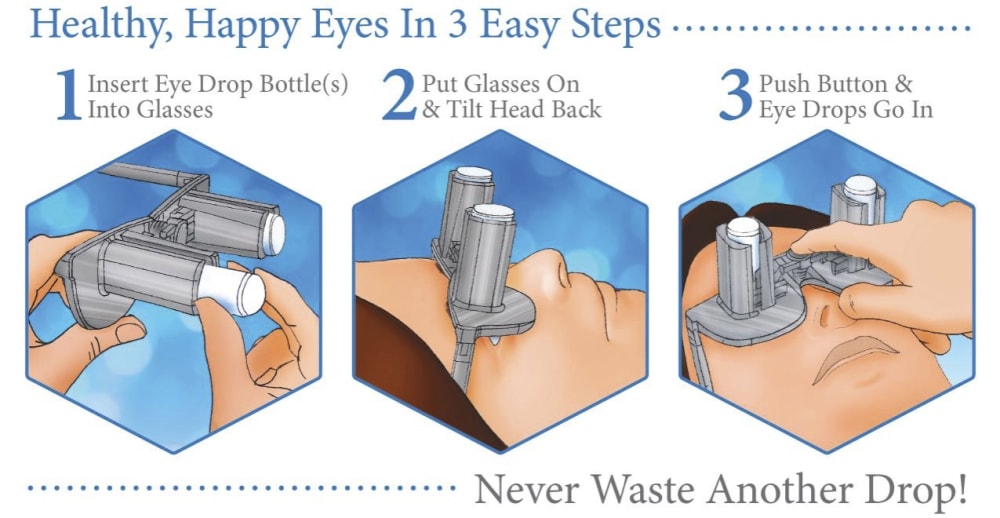
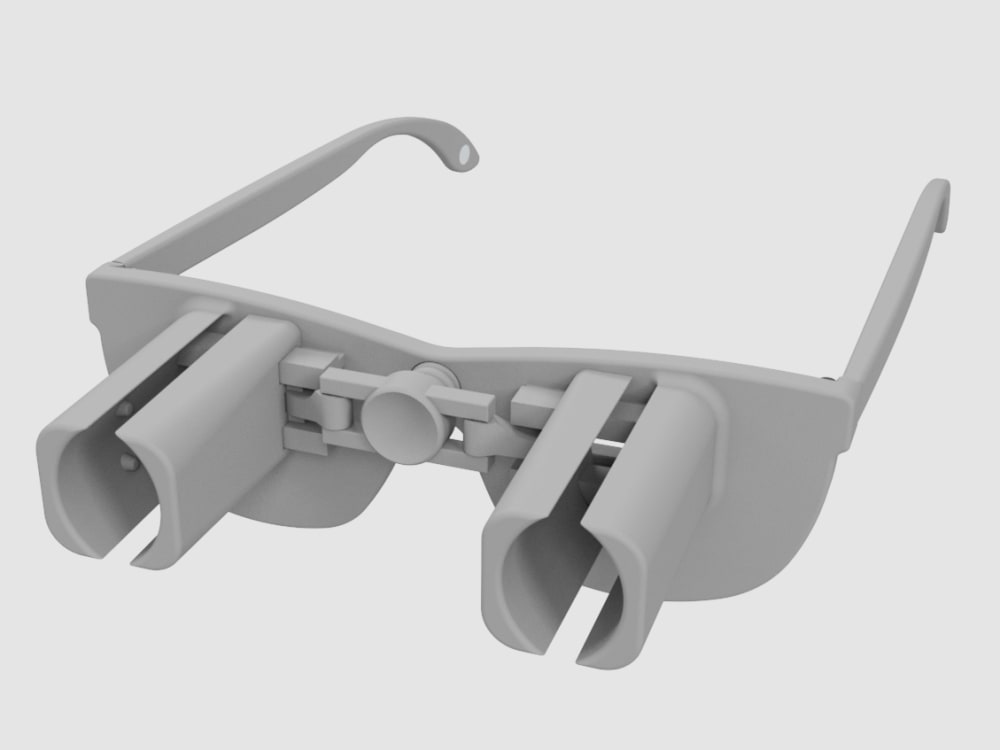
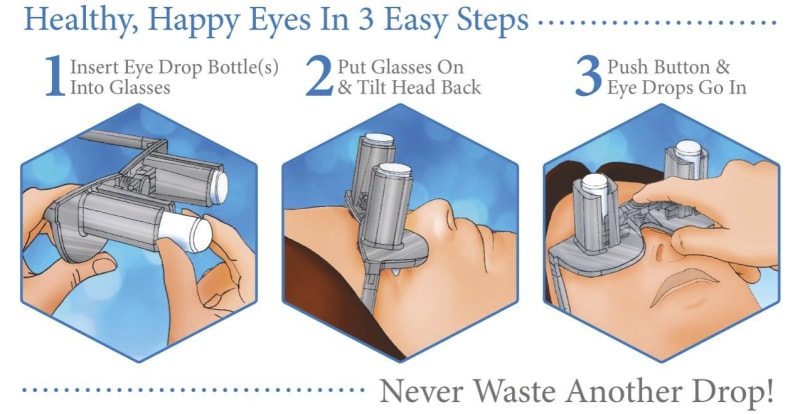
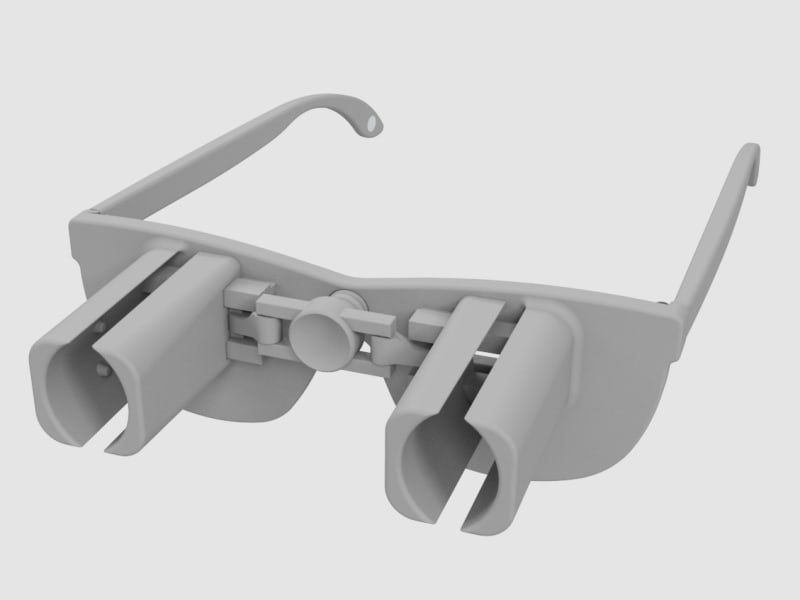
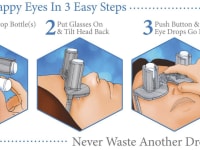
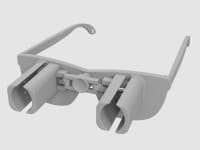
A revolutionary new way to apply eye drops with the simple push of a button. This eye drop device holds, aligns, and administers eye drops simultaneously providing relief for the millions requiring eye drops for cataracts, glaucoma, LASIK, and dry eye.
Self-administering eye drops is difficult for multiple reasons. The patient must hold the eye drop bottle steadily above their head, align it appropriately with respect to their eye, and squeeze the bottle. Many patients, particularly the elderly, struggle with hand tremors making it difficult to hold the bottle steady above their head and align it with their eye. Patients find it challenging to squeeze the bottle with the correct amount of force resulting in either an inadequate or excessive amount of eye drop solution. Some experience apprehension when dropping something into their eye. When inserting eye drops must be repeated multiple times daily, it can become a burdensome reality. Improper administration can lead to increased ocular pathology, slower healing time, and wasted drops and money.
Alternatively to self-administration, eye drop bottle aligning or squeezing devices exist. However, a detailed prior art/market search shows that no current design eliminates all of the difficulties.
Our eye drop device solves all of the primary difficulties experienced in administering eye drops by simultaneously holding the bottle(s), aligning the bottle(s) over the eye, and administering the drops/squeezing the bottles for you. The ability of the device to eliminate all three difficulties simultaneously with simplified user interaction makes it a novel design. Drops can be administered in both eyes concurrently, a capability not found anywhere in the market. This invention improves the patients’ eye drop experience allowing for improved post-operative care, better compliance, decreased eye disease, and reduced eye drop waste.
There is a well-defined and significant market for the device. In the US alone, an estimated 38 million people will be diagnosed with Cataracts by 2030, a number steadily increasing to 50 million by 2050 as the population ages (National Eye Institute). Patients are required to take approximately 130 drops per eye during the month following Cataract surgery. Over 22 million Americans are diagnosed with Glaucoma and Dry Eye Disease (American Academy of Ophthalmology), both requiring multiple drops daily for treatment. 12 million Americans treated with LASIK require drops for post-operative care, as well as lubrication drops for dryness.
The device can be 3D printed or injection molded. It will be made from a durable polymer, like nylon. It has been designed to be 3D printed in several pieces then assembled. 3D printed prototypes were evaluated for functionality with different size bottles confirming easy implementation. 3D printing may prove cost effective and requires minimal upfront capital. At scale, injection molding would provide lower per part cost, but requires initial capital to design and fabricate the injection molds. Further prototypes and analysis will refine functionality over the range of bottle sizes. The device could be packaged with post-operative kits for eye surgeries, sold in pharmacies, online, or directly from the doctor.
Like this entry?
-
About the Entrant
- Name:Robert Fowler
- Type of entry:teamTeam members:Robert M Fowler
Marc P Otto, O.D. - Software used for this entry:Autodesk Fusion 360
- Patent status:none