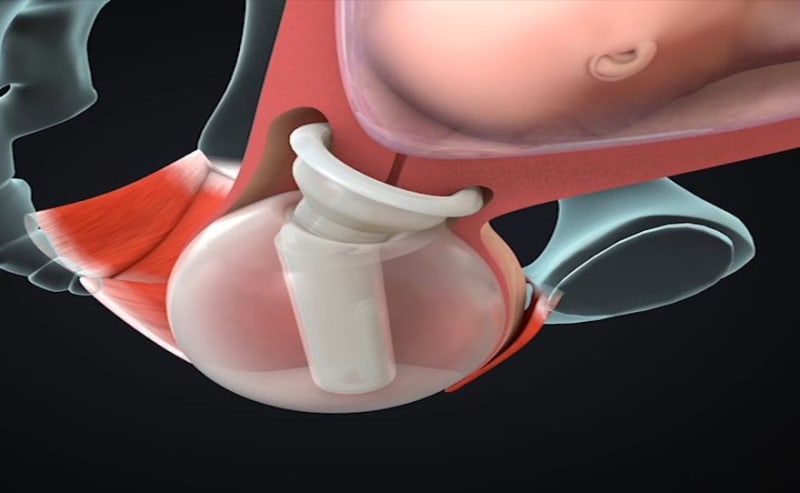
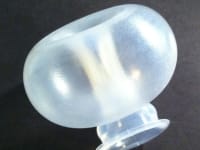
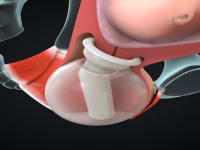
ViaTechMD has invented and developed the first and only device-based treatment for various conditions known to contribute to preterm birth (“PTB”).
PTB is a growing global healthcare crisis and is the leading cause of infant death and morbidity within the first months of life. Nearly 3,000 babies die EACH DAY as a consequence of PTB which is also recognized as one of the most significant contributing factors in several lifelong crippling diseases including autism, cerebral palsy, blindness, deafness, various respiratory ailments, and other cognitive and learning disabilities.
The latest publication from WHO (2018) reports that in 2014, approximately 10.6% of all live births globally were preterm. Every year, an estimated 15 million babies are born preterm (before 37 completed weeks of gestation), and that number is rising. Socioeconomic consequences of PTB are estimated to exceed $52 billion each year.
ViaTechMD's globally patented device, the Cervical Stabilization Device (“CSD”), offers a cost effective, non-invasive, medication-free, preventative treatment designed to support a successful and natural birth by addressing a wide range of conditions known to contribute to PTB (such as cervical incompetence, known cases of organ and tissue compromise, multiple fetus pregnancies, late-in-life pregnancies, and others).
Successful CSD human fitment studies took place in December of 2016. The generation 6 CSD, in its near-commercial form, will undergoing a second round of human fitment studies this coming July, ahead of pivotal FDA clinical trials that are expected to commence in the fourth quarter of this year.
Current treatments in prevention of PTB include cervical cerclage (a costly, high-risk, invasive surgical procedure) and use of progestins (natural or synthetic female hormones that present have a number of serious risks particularly to male fetuses). Neither treatment is effective in reducing bearing forces acting upon the uterine and cervical tissues that are known to accelerate the birthing, effacement and dilation processes. And, after decades of practice neither treatment has demonstrated proof of efficacy.
To date, ViaTechMD has been operated as a virtual company and has therefore been very capital efficient. The Company has raised approximately $3.5 million in "friends and family" equity capital and is seeking additional equity capital to fund a full-time management team, finalize product development, establish manufacturing operations in partnership with Freudenberg Medical and to conduct a pivotal FDA clinical trial.
To learn more, please visit the ViaTechMD website at viatechmd.org, and be sure to watch both video animations at the bottom of the homepage.
Thank You.
-
Awards
-
 2019 Medical Category Winner
2019 Medical Category Winner -
 2019 Top 100 Entries
2019 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Benjamin Booher, Sr.
- Type of entry:individual
- Patent status:patented