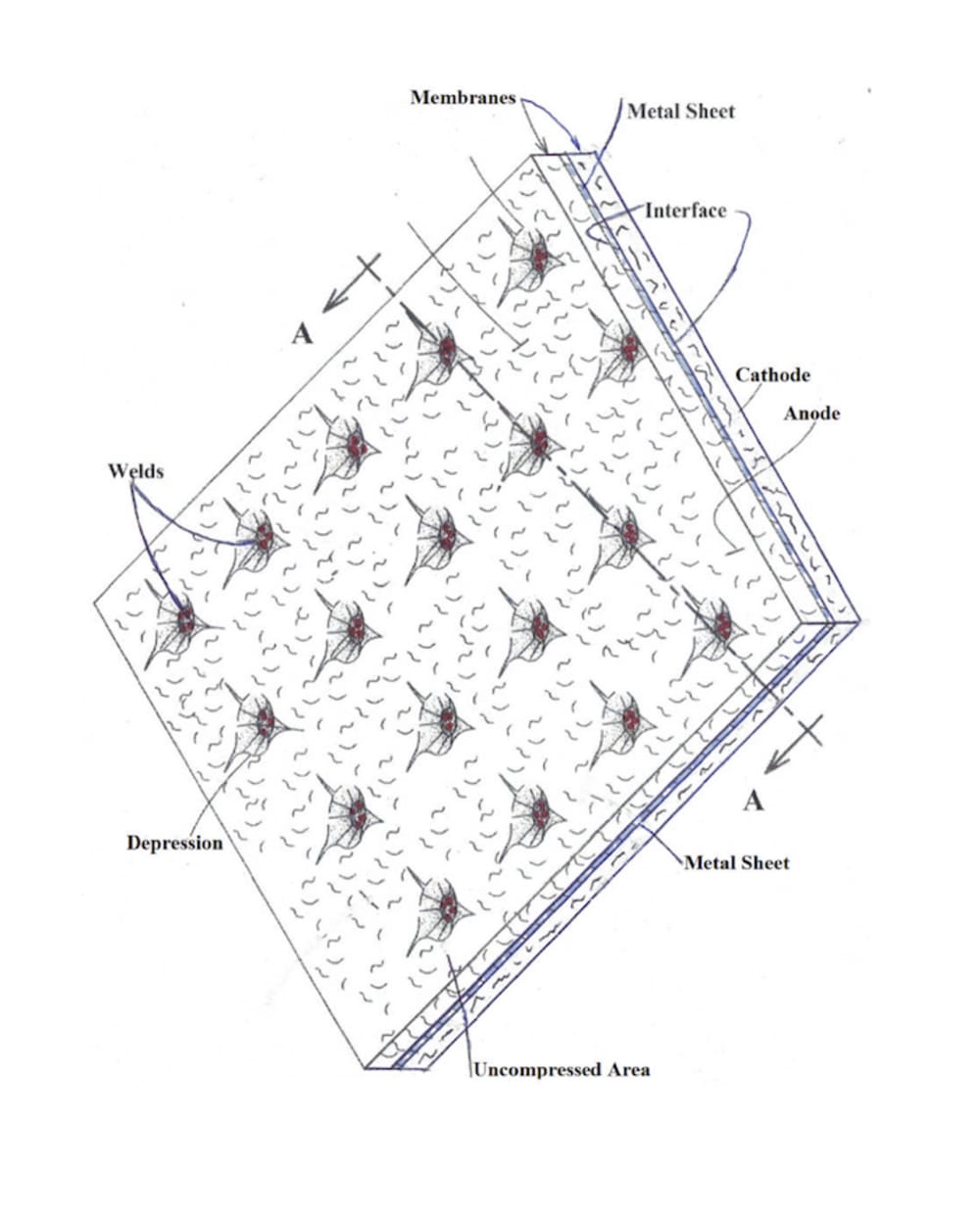
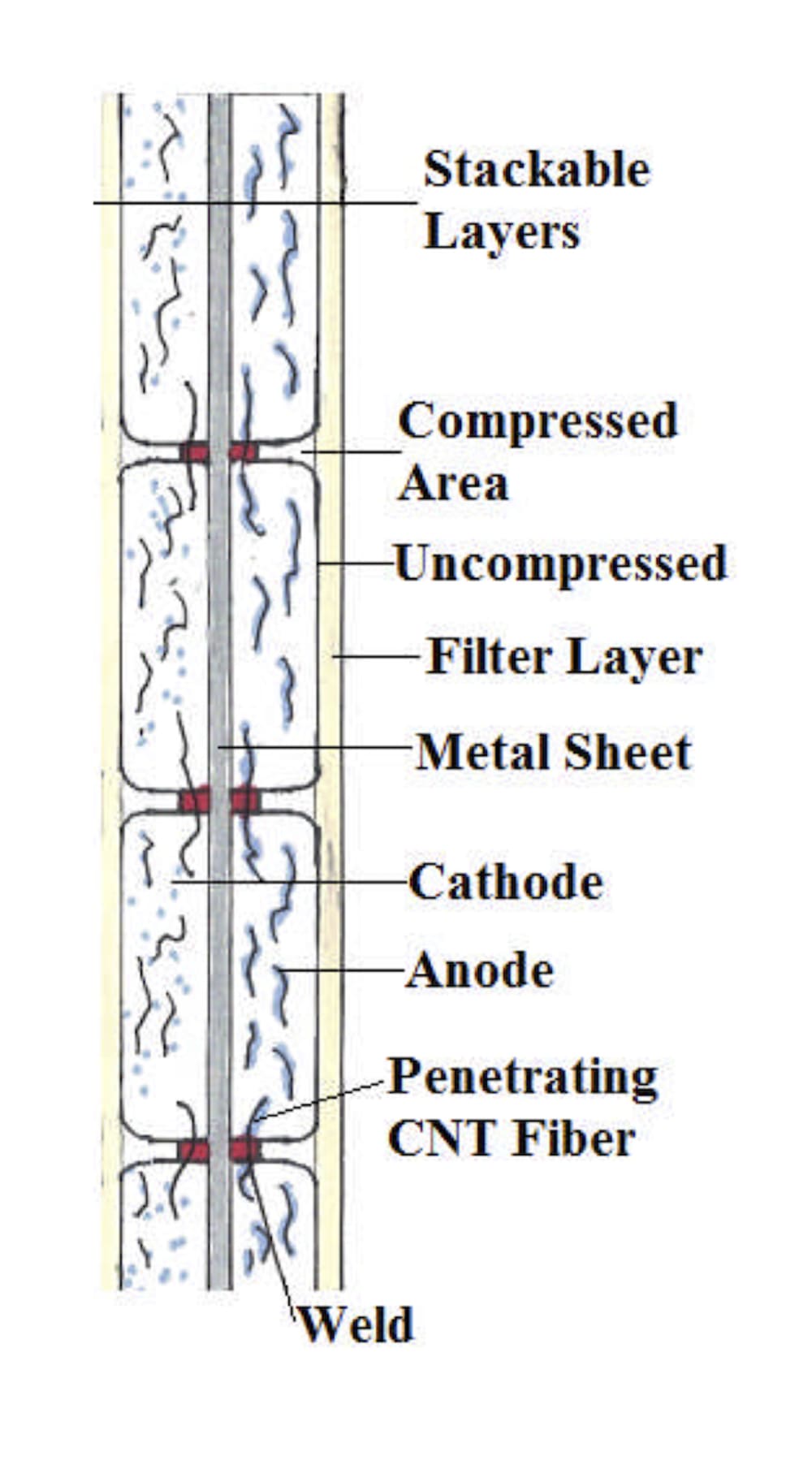
Present galvanic electrode architecture is characterized by low electrode area specific current density and long battery recharge interval. Factors limiting secondary battery current may be ion exchange between electrodes and/or electron impedance between solid phase coatings and metal current collectors. A new approach to electrode structure replaces contact resistance in the interface separating the solid phase and adjacent metal with alternative conduction pathways to greatly lower resistance to electron flow. Electrodes comprise a cohesive carbon membrane of nonwoven CNT or graphene sheet, loaded with virtually any faradaic or catalytic chemistry as a carbon surface coating or lodged and immobilized by carbon fiber. Examples are widely reported in the literature. Membranes attached as pellicles to a metal surface in accordance with the invention have virtually 0 contact resistance at a plurality of closely spaced locations. Electron conduction to these 'sinks' is in-plane along CNT fibers for short distances within the active energy storing material. Solid phase ohmic resistance can be computed on the basis of double layer (DL) capacitance as less than 0.5ρT Ω-cm^2. T is membrane thickness. Material in-plane resistivity, ρ was tested at 0.01 Ω-cm but can be somewhat higher for use in battery electrodes. The disputed influence of ion kinetics in secondary batteries can now be more directly measurable given ultralow solid phase impedance. Present high resistance in galvanic cell electrodes causes i^2R heat and hazard, limits permissible current density which extends recharge time and limits cell power. Novel fuel cell design using ultralow resistance electrodes will enable direct complete oxidation of most hydrocarbon fuels with three times the efficiency of CARNOT cycle internal combustion engines (ICE) .
Proof of concept for these electrodes is trivially obvious when assembled as an electrochemical capacitor. Testing of e.g. LIB requires relevant chemistry. Either can be tested with applied pressure using the illustrated separator. High volume manufacturing can be accomplished with appropriate tooling to produce welded contact points, also illustrated.
Clearly, once the concept is verified, galvanic cells will have to adopt this electrode architecture to compete in the industry. That is the very definition of transformative technology. We believe natural gas fuel cells will power charging stations at megawatt levels using abundant natural gas. Batteries will store that energy in road vehicles because full recharge can be accomplished in a few minutes instead of hours. The reason is simple. Present LIB can charge at less than 10 milliamps/cm^2. An ultralow impedance electrode can charge at 200 or more milliamps/cm^2. Two hours divided by 20 is 7 minutes.
-
Awards
-
 2017 Top 100 Entries
2017 Top 100 Entries
Like this entry?
-
About the Entrant
- Name:Halbert Fischel
- Type of entry:teamTeam members:Halbert Fischel, Prof.-Dr. Philip Lubin, Brian Clark
- Software used for this entry:Matlab
- Patent status:patented