The modern vehicles operate with lead-acid batteries, which are not environmentally correct, beyond the fact that they are expensive and have toxic products (sulfuric acid and the lead itself). In this work, a new idea is proposed in order to replace such electrochemical systems with new compounds. This new type of battery would be an aluminum-copper battery. This system has several advantages when compared with the classical lead-acid batteries:
1. Aluminum is a fully recyclable metal. The idea here is to use it from renewable sources and materials already existent, as per example: cans, electronic components wasted, etc., without being necessary to produce more aluminum from their ores, by saving energy and natural resources.
2. Copper is also a very known and non-toxic metal, and can be completely reused from several materials. It is present in a wide range of modern applications, which becomes it an easy metal to be recycled.
3. Both metals have lesser densities than the lead (11300 kg/m3): aluminum: 2700 kg/m3; copper: 8920 kg/m3 [1]. This makes the battery lighter and easier to handle.
4. The solutions of the electrolyte may be constituted of sulfates or chlorides: Al2(SO4)3 or AlCl3; CuSO4 or CuCl2. This is an advantage because in the classical batteries the electrolyte is sulfuric acid, which is dangerous and highly corrosive.
From the literature [2], the semi-reactions of electrochemical reduction of aluminum and copper are described below:
Al+3 + 3e-> Al(s) E = -1.68 V (1)
Cu+2 + 2e-> Cu(s) E = 0.34 V (2)
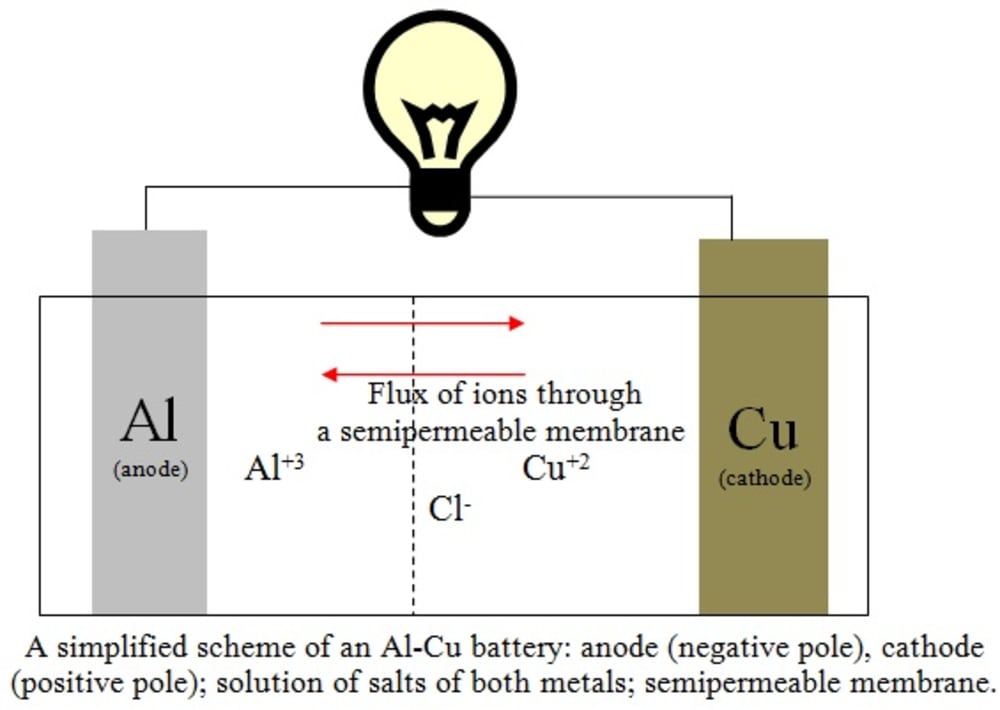
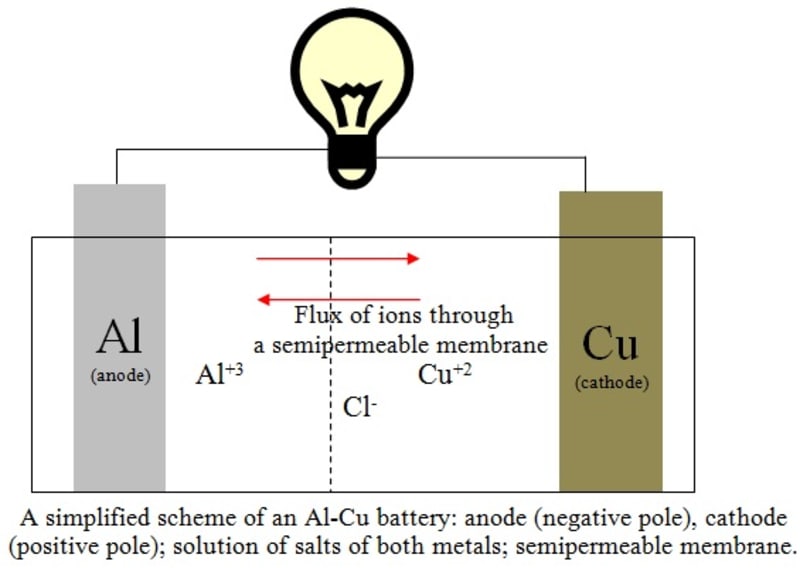
The aluminum has a lesser reduction potential, in order that it is oxidized; so the reaction (1) must be inverted and both reactions must be added to give the final reaction:
2Al(s) + 3Cu+2 -->2 Al+3 + 3Cu(s) E = 2.02 V (3)
A scheme of the reaction (3) is illustrated in the figure. The flux of ions occurs through a semipermeable membrane. The multiplication of the Equation (1) by 2 and the Equation (2) by 3 was carried out to account correctly for the total number of 6 moles of electrons replaced in the process. A full battery Al-Cu would provide about 12 V of electrical potential if connected six pairs in series, which is the same potential obtained with a lead-acid battery, but giving additional advantages: cheaper costs of manufacturing due to its simplicity of operation when compared with the car batteries where PbO2 is also present, higher durability and higher currents (and thus, lesser internal electrical resistance) due to greater ionic mobility of the chloride ions.
References
[1] Patnaik, P. Handbook of Inorganic Chemicals, McGraw-Hill, 2002.
[2] Brett, C.M.A., Brett A.M.O. Electrochemistry – Principles, Methods and Applications, Oxford University Press, 1993.
Like this entry?
-
About the Entrant
- Name:Cleiton Porciuncula
- Type of entry:individual
- Patent status:none